About Magellan Stem Cells

Magellan, a private Australian company, is a global leader in the research, development and manufacture of stem cells.
Magellan has been researching and manufacturing human stem cells since 2013, following successful preclinical work in the use of stem cells for the treatment of osteoarthritis in animals.
Magellan has cultured more than 400 billion mesenchymal stem cells.
Magellan manufactures autologous (patient’s own cells) and is trialling allogeneic (donor) stem cells for the treatment of osteoarthritis.
Magellan’s research specialises in the treatment of osteoarthritis, a debilitating condition affecting more than 500 million people across the globe.
Magellan has received approval to operate a GMP Laboratory and the TGA has issued Magellan a licence to manufacture allogeneic (donor) mesenchymal stem cells for Magellan’s upcoming trials.
Our vision is to enrich the lives of people across the globe who struggle with osteoarthritis, by significantly reducing pain and improving mobility.
Directors and executive team
Board of Directors
 Magelan’s Non-Executive Chairman, Mr Lou Panaccio is a Chartered Accountant with more than 35 years of executive leadership experience in healthcare services and life sciences, including more than 25 years of board-level experience and a wealth of executive management experience in business and healthcare services. Mr Panaccio has acted as the Chief Executive Officer of Monash IVF, Melbourne Pathology and Executive Chairman of Health Networks Australia. He is currently Chairman of AVITA Medical Limited (ASX and Nasdaq Listed), Chairman of Adherium Limited (ASX), Chairman of Unison Housing Limited, and Non Executive Director of Mecwacare Limited. Mr Panaccio retired as a Non Executive Director of Sonic Healthcare Limited and Rhythm Biosciences Limited in 2024.
Magelan’s Non-Executive Chairman, Mr Lou Panaccio is a Chartered Accountant with more than 35 years of executive leadership experience in healthcare services and life sciences, including more than 25 years of board-level experience and a wealth of executive management experience in business and healthcare services. Mr Panaccio has acted as the Chief Executive Officer of Monash IVF, Melbourne Pathology and Executive Chairman of Health Networks Australia. He is currently Chairman of AVITA Medical Limited (ASX and Nasdaq Listed), Chairman of Adherium Limited (ASX), Chairman of Unison Housing Limited, and Non Executive Director of Mecwacare Limited. Mr Panaccio retired as a Non Executive Director of Sonic Healthcare Limited and Rhythm Biosciences Limited in 2024.
 Mr Peter Hansen has more than 30 years’ experience in founding and leading four biotechnology and medical related companies (Australia, USA and Asia). He has chaired four public companies (Australia and UK) and three IPO’s. His focus on marketing, manufacturing technologies/costs (Australia, Singapore, Malaysia), research collaborations and international licensing.
Mr Peter Hansen has more than 30 years’ experience in founding and leading four biotechnology and medical related companies (Australia, USA and Asia). He has chaired four public companies (Australia and UK) and three IPO’s. His focus on marketing, manufacturing technologies/costs (Australia, Singapore, Malaysia), research collaborations and international licensing.
 Mr Ross Williams is a Chartered Accountant, MBA with over 30 years’ Finance and Accounting experience in Multinational Public and Private Companies, including 10 years as GM of a leading Allied Health company. He is a director of Melbourne Stem Cell Centre Research and Australian Osteoarthritis Clinic.
Mr Ross Williams is a Chartered Accountant, MBA with over 30 years’ Finance and Accounting experience in Multinational Public and Private Companies, including 10 years as GM of a leading Allied Health company. He is a director of Melbourne Stem Cell Centre Research and Australian Osteoarthritis Clinic.
 Mr Michael Kenihan is a Director at Melbourne Stem Cell Centre Research and Australian Osteoarthritis Clinic. He is a past president and CEO of Sports Medicine Australia and a qualified Sports Physiotherapist. Previously, he served as general manager of Lifecare, a leading ASX-listed allied health company.
Mr Michael Kenihan is a Director at Melbourne Stem Cell Centre Research and Australian Osteoarthritis Clinic. He is a past president and CEO of Sports Medicine Australia and a qualified Sports Physiotherapist. Previously, he served as general manager of Lifecare, a leading ASX-listed allied health company.
 Associate Professor Julien Freitag is an experienced musculoskeletal specialist and a fellow of the Australasian College of Sport and Exercise Physicians. His practice is focused on the active management of musculoskeletal conditions including osteoarthritis and tendinopathy. Dr Freitag’s award-winning research is internationally regarded.
Associate Professor Julien Freitag is an experienced musculoskeletal specialist and a fellow of the Australasian College of Sport and Exercise Physicians. His practice is focused on the active management of musculoskeletal conditions including osteoarthritis and tendinopathy. Dr Freitag’s award-winning research is internationally regarded.
Scientific, Quality and Operations team
Chief Medical Officer
 Associate Professor Julien Freitag is an experienced musculoskeletal specialist and a fellow of the Australasian College of Sport and Exercise Physicians. His practice is focused on the active management of musculoskeletal conditions including osteoarthritis and tendinopathy.
Associate Professor Julien Freitag is an experienced musculoskeletal specialist and a fellow of the Australasian College of Sport and Exercise Physicians. His practice is focused on the active management of musculoskeletal conditions including osteoarthritis and tendinopathy.
Dr Freitag’s research is internationally regarded and has been awarded the Osteoarthritis and Cartilage Open Clinical Research Publication of the Year Award for the excellence of its original research. Osteoarthritis and Cartilage Open is the open access journal of Osteoarthritis Research Society International (OARSI).
Dr Freitag holds an adjunct Associate Professor position at Charles Sturt University in the School of Rural Medicine. Dr Freitag has been a selection committee member of the NHMRC MRFF 2020 Stem Cell Therapies Mission Grant Application Committee.
He is an active member of the Osteoarthritis Research Society International (OARSI) and a member of their Regenerative Medicine in Osteoarthritis Discussion Group.
 Dr Kiran Shah is an accomplished head scientific officer (HSO) and head of laboratory operations with more than 20 years’ experience in molecular virology, stem cell research, cellular therapy, clinical trial management, and biotechnology innovations.
Dr Kiran Shah is an accomplished head scientific officer (HSO) and head of laboratory operations with more than 20 years’ experience in molecular virology, stem cell research, cellular therapy, clinical trial management, and biotechnology innovations.
Dr Shah has proven expertise in leading high-impact R&D projects, strategic oversight of clinical trials, and translating cutting-edge research into commercialised therapies.
She is adept at liaising with regulatory bodies, driving GMP-compliant processes, and spearheading scientific innovation to enhance patient outcomes.
Dr Shah has shown strong leadership in fostering cross-functional teams, securing funding, and advancing intellectual property (IP) portfolios to position the company at the forefront of biotechnology.
Dr Shah holds a PhD in Biochemistry and Molecular Biology, from Swinburne University in collaboration with Royal Children’s Hospital, post doc at CSL, complemented by an Executive Master of Business Administration. She brings a unique blend of scientific expertise and strategic leadership to her work in clinical development and pharmaceutical manufacturing.
 Dr Nirali Shah earned her PhD from Swinburne University in 2014, specialising in umbilical cord-derived mesenchymal stem cells (MSCs) for clinical applications. With a deep passion for advancing stem cell-based therapies, she is dedicated to developing innovative models for treating arthritic conditions and uncovering novel therapeutics.
Dr Nirali Shah earned her PhD from Swinburne University in 2014, specialising in umbilical cord-derived mesenchymal stem cells (MSCs) for clinical applications. With a deep passion for advancing stem cell-based therapies, she is dedicated to developing innovative models for treating arthritic conditions and uncovering novel therapeutics.
Bringing over a decade of expertise in stem cell research, Nirali has honed her skills in method development, cell characterisation, and cutting-edge immunological and molecular analytical techniques. She is particularly fascinated by emerging cell therapy technologies, including 3D cell culture, and is committed to driving advancements that enhance treatments for osteoarthritic diseases.
Nirali’s expertise extends to GMP training, where she is actively engaged in process validation and refining methods to ensure the safe and effective application of stem cell therapies. Her work is at the forefront of regenerative medicine, pushing the boundaries of what’s possible in cellular therapeutics.
Masters in Laboratory Technology
 With extensive expertise in stem cell culture techniques, Teena George is a highly skilled scientist specialising in the processing, collection, and expansion of stem cells from lipoaspirate tissues. Her expertise spans bioassays and advanced analytical methods, including ELISA, PCR, and protein purification, making her a key contributor to cutting-edge research in regenerative medicine.
With extensive expertise in stem cell culture techniques, Teena George is a highly skilled scientist specialising in the processing, collection, and expansion of stem cells from lipoaspirate tissues. Her expertise spans bioassays and advanced analytical methods, including ELISA, PCR, and protein purification, making her a key contributor to cutting-edge research in regenerative medicine.
Teena has played a pivotal role in maintaining clinically derived stem cell cultures, overseeing the harvesting and cryopreservation of stem cells, and ensuring rigorous quality control through meticulous documentation. She brings a wealth of experience in cell counting, cryopreservation, and preparing clinical stem cell dosages with precision and care.
Committed to advancing best practices in cell therapy, Teena has undergone GMP (good manufacturing practice) uplift training and is involved in method validation and documentation, ensuring compliance with industry standards.
 With a wealth of experience in GMP Quality Management, David Petrie is a seasoned leader in quality assurance and regulatory affairs. Having held senior roles such as Senior Quality Manager at Pfizer and Quality Director at PCI Pharma Services, he brings invaluable expertise to Magellan’s development of a Contract Development and Manufacturing Organisation (CDMO).
With a wealth of experience in GMP Quality Management, David Petrie is a seasoned leader in quality assurance and regulatory affairs. Having held senior roles such as Senior Quality Manager at Pfizer and Quality Director at PCI Pharma Services, he brings invaluable expertise to Magellan’s development of a Contract Development and Manufacturing Organisation (CDMO).
David’s deep knowledge of GMP compliance, aseptic manufacturing, and regulatory frameworks ensures that Magellan meets the highest industry standards. He has successfully led facility GMP inspections with both TGA and international regulatory authorities, demonstrating his ability to navigate complex regulatory landscapes with precision and confidence.
 Nick is the head of Operations at Magellan stem cells. Prior to this role, Nick has worked in multiple senior leadership roles at both Pharma and Medical device companies. First as the head of Supply Chain Management at Medical development international (ASX MVP), where he managed the Production and Supply chain of the company’s major product Penthrox both in Australia and International. More recently as the head of Operations at Universal biosensors, where he managed major business functions including, Operations, Supply Chain, Quality, Regulatory affairs, Engineering & Clinical, both in Australia and overseas.
Nick is the head of Operations at Magellan stem cells. Prior to this role, Nick has worked in multiple senior leadership roles at both Pharma and Medical device companies. First as the head of Supply Chain Management at Medical development international (ASX MVP), where he managed the Production and Supply chain of the company’s major product Penthrox both in Australia and International. More recently as the head of Operations at Universal biosensors, where he managed major business functions including, Operations, Supply Chain, Quality, Regulatory affairs, Engineering & Clinical, both in Australia and overseas.
About osteoarthritis
Osteoarthritis, a painful and debilitating condition, affects 100 million people in the developed world and more than 500 million people globally.
Osteoarthritis is the most common chronic joint disease in Australia and one of the leading causes of pain, disability and early retirement.
Osteoarthritis has been declared a national priority in Australia and is a leading global unmet clinical need.
The cost to developed economies and to patients is surging and growing rapidly.
In Australia alone, at least 2.2 million people aged 25-to-64 are affected by arthritis.
The burden on the global economy is more than $A23.9 billion every year in direct and indirect costs. This figure is projected to increase by 58 per cent by 2032.
Current treatments for osteoarthritis have poor efficacy. There is limited short-term pain improvement, no disease modification and the potential risk of significant side effects.
Osteoarthritis affects 20 per cent of the population aged 45 and over, and 80 per cent of people aged 65 and over.
The condition is a significant healthcare burden on individuals and society, costing more than 1 per cent of annual GDP in developed nations.
The average non-surgical medical cost per patient is estimated at $US5,900 a year.
Should you have osteoarthritis we suggest that you contact Magellan’s research collaboration partner Melbourne Stem Cell Centre Research for more information.
Frequently asked questions
Magellan holds a GMP (good manufacturing practice) licence from the Australian regulator, the TGA (Therapeutic Goods Administration) and can manufacture mesenchymal stem cells (MSC’s) under GMP conditions to be used for trials. Magellan is also working closely with regulators in the US, EU and UK.
Magellan manufactures autologous (patient’s own) and allogeneic (donor) mesenchymal stem cells (MSCs). MSCs have the ability to self-renew and also exhibit multilineage differentiation. MSCs can be isolated from a variety of tissues, with Magellan isolating MSCs from adipose tissue. Magellan, via its collaboration partner Melbourne Stem Cell Centre Research, has seen autologous cells used to treat more than 1500 osteoarthritis patients.
Since 2013, more than 1500 patients have been treated for OA using Magellan-manufactured autologous and allogeneic stem cell lines. Magellan, with its research collaboration partner Melbourne Stem Cell Centre Research, has published in several prestigious international medical journals. Overviews and links to the research are available on the Research page.
Magellan research is ongoing. Following the results of its Phase I/II allogeneic OA trial, these results were published in Osteoarthritis and Cartilage Open. Magellan is planning further research, including a Phase III allogeneic OA trial.
Since 2013, more than 1500 patients have been treated for OA using Magellan-manufactured autologous mesenchymal stem cell (MSC) lines. Magellan’s optimum MSC cell lines have been selected based on superior patient clinical outcomes and detailed genetic and DNA analysis of the characteristics of these optimum cell lines.
According to the National Health Survey 2017-2018, more than 2.2 million Australians suffered with OA in 2016.
One in five Australians aged over 45 have OA.
Although OA affects people of all ages, the prevalence increases sharply from 45 years, with up to 80 per cent of people over the age of 65 having radiological (scans) evidence of OA.
More than 500 million people across the world suffer from OA, which is recognised as a global unmet clinical need.
Magellan does not treat patients directly, but does supply the MSC’s to be used for OA patient treatments. For those seeking more information on OA treatment, please contact our collaborative clinical research partner, Melbourne Stem Cell Centre Research.
Magellan’s successful Phase I/II allogeneic OA trial results (2020) showed:
- long-term pain reduction;
- significant functional activity improvement; and
- evidence of osteoarthritis disease modification, cartilage regrowth, delay in OA progression with the potential to defer joint replacement surgery.
The results of the Phase I/II trial were published in Osteoarthritis and Cartilage Open.
Since 2013, Magellan – in partnership with Melbourne Stem Cell Centre Research – has undertaken a lengthy research and development pathway including clinical trial programs and, critically, real-world treatment case studies/series on the use of MSC’s for OA.
This work was based on Magellan’s preclinical research evidence in 2010 with veterinary osteoarthritis treatments. Magellan is well advanced in its medical and scientific product development strategy. This is supported by strong financial and corporate governance programs in order to commence its pivotal Phase III allogeneic OA trial in 2025.
Magellan’s plan is to continue to develop the company from a focus on R&D to a full scale diverse product commercialisation company across global markets.
Based on a successful Phase III allogeneic OA trial outcome, Magellan plans to make an application to the TGA for product approval and registration in Australia in 2028.
Magellan has partnered/ conducted extensive preclinical research. Its research partners have included: Australian Veterinary Stem Cell P/L; Swinburne University of Technology: Hudson Institute; and Monash Vet Clinic. A summary of the preclinical research is published in Stem Cells International.
To be confirmed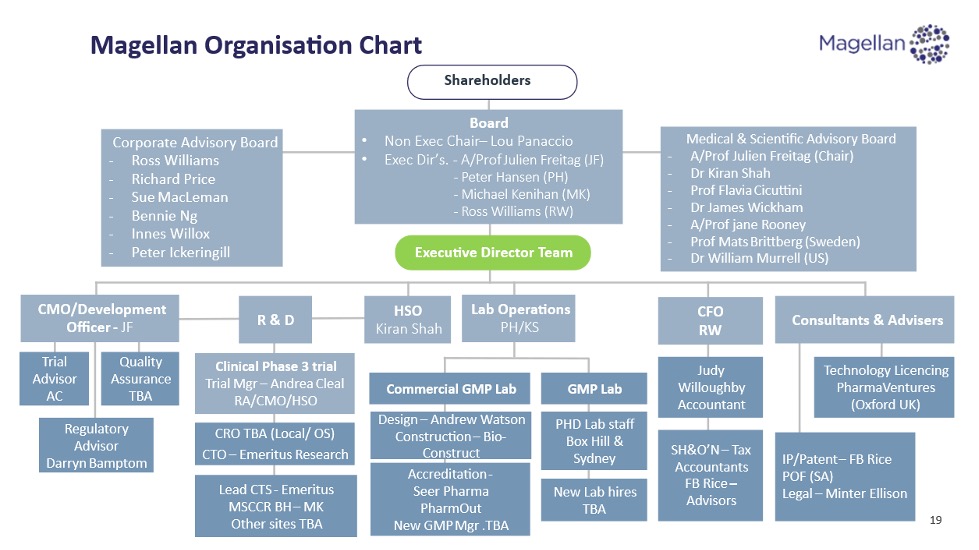
Magellan is a private Australian company. It first raised Capital in 2012 to commence the supply of autologous MSC’s to clinicians in Australia and New Zealand.
Magellan then raised further share capital in 2022 and 2023 to help fund its program of Phase I/II allogeneic OA trials, construct, expand and equip its GMP lab capabilities, commence overseas regulatory processes and develop its in-house management team.
Further completed Capital raise programs in 2024 and 2025 will enable Magellan to progress to the commencement of its pivotal Phase III allogeneic OA trial in 2025 and further develop its GMP lab and in-house management team resources.
