Magellan Stem Cells: pioneering osteoarthritis treatment
Magellan is investing in a future where osteoarthritis sufferers can move freely and live pain-free
Magellan’s MAG200 donor stem cell product aims to revolutionise osteoarthritis care, bringing relief to millions of people worldwide who struggle with joint pain and mobility challenges.
Magellan is researching and developing new treatments for osteoarthritis, a condition that causes pain and restricts movement. OA affects more than 500 million people globally with annual costs in the developed world estimated at more than $A23.9 billion and growing.
Magellan Stem Cells holds a good manufacturing practice (GMP) laboratory licence from the Therapeutic Goods Administration (TGA) to manufacture mesenchymal stem cells and is a global leader in the research, development, and manufacture of stem cells.
Magellan has developed an efficient, cost-effective stem cell culturing process and gained unprecedented scientific insights into the specific features of different stem cell lines and the unique characteristics of the genetic profile of the most efficacious mesenchymal stem cell lines for the treatment of osteoarthritis.
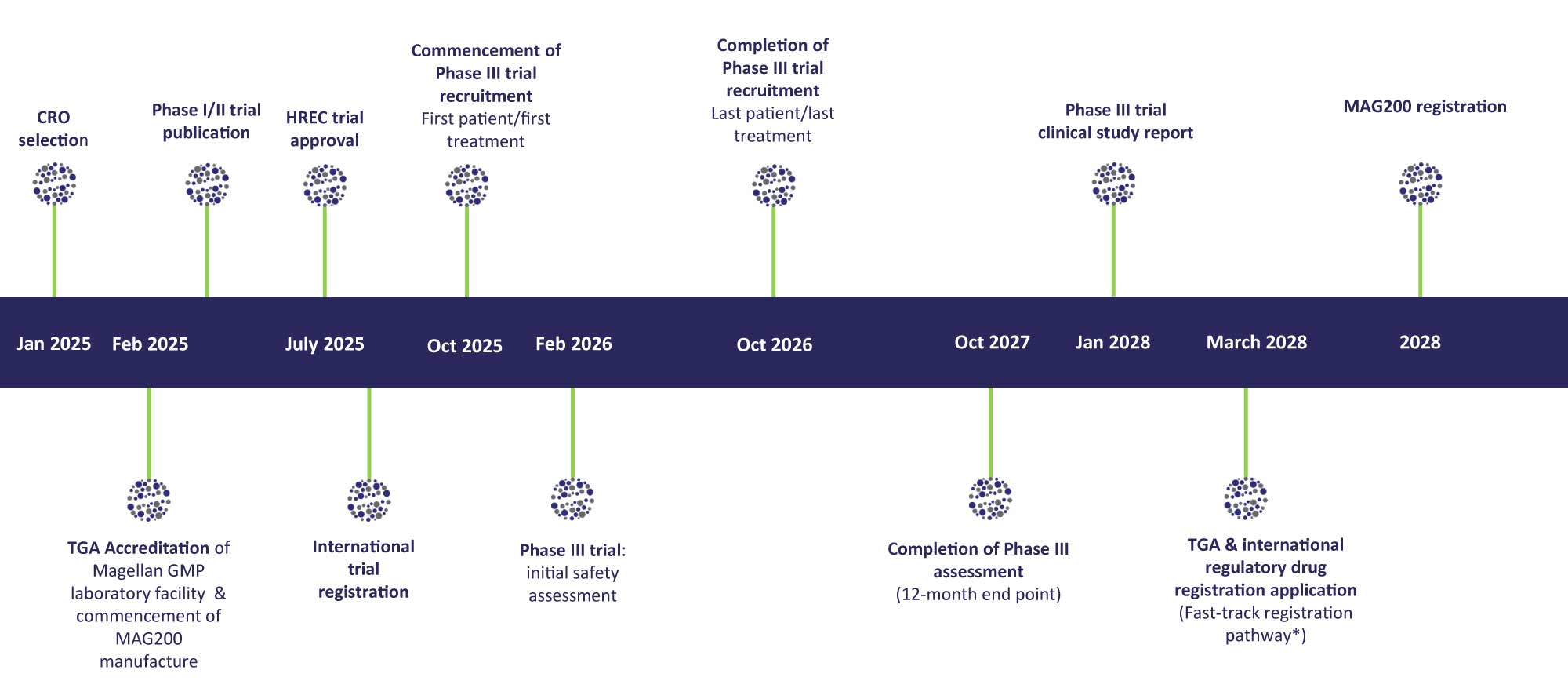
Key Development Milestones
2025 to 2028
NB. The above timeline is indicative and illustrative only and is not a forecast or projection or any assurance or guarantee that the indicated timelines will be met or that any individual milestone will be achieved in whole or in part. Refer to the Company’s ‘Important Notice and Disclaimer’ (Page 1 & 2).
*Fast Track Regulatory Pathways anticipated due to recognised ‘unmet clinical need’
About osteoarthritis
Osteoarthritis, a painful and debilitating condition, affects 100 million people in the developed world and more than 500 million people globally.
Osteoarthritis is the most common chronic joint disease in Australia and one of the leading causes of pain, disability and early retirement.
Osteoarthritis has been declared a national priority in Australia and is a leading global unmet clinical need.
The cost to developed economies and to patients is surging and growing rapidly.
In Australia alone, at least 2.2 million people aged 25-to-64 are affected by arthritis.
The burden on the global economy is more than $A23.9 billion every year in direct and indirect costs. This figure is projected to increase by 58 per cent by 2032.
Current treatments for osteoarthritis have poor efficacy. There is limited short-term pain improvement, no disease modification and the potential risk of significant side effects.
Osteoarthritis affects 20 per cent of the population aged 45 and over, and 80 per cent of people aged 65 and over.
The condition is a significant healthcare burden on individuals and society, costing more than 1 per cent of annual GDP in developed nations.
The average non-surgical medical cost per patient is estimated at $US5,900 a year.
Should you have osteoarthritis we suggest that you contact Magellan’s research collaboration partner Melbourne Stem Cell Centre Research for more information.
Frequently asked questions
Magellan holds a GMP (good manufacturing practice) licence from the Australian regulator, the TGA (Therapeutic Goods Administration) and can manufacture mesenchymal stem cells (MSC’s) under GMP conditions to be used for trials. Magellan is also working closely with regulators in the US, EU and UK.
Magellan manufactures autologous (patient’s own) and allogeneic (donor) mesenchymal stem cells (MSCs). MSCs have the ability to self-renew and also exhibit multilineage differentiation. MSCs can be isolated from a variety of tissues, with Magellan isolating MSCs from adipose tissue. Magellan, via its collaboration partner Melbourne Stem Cell Centre Research, has seen autologous cells used to treat more than 1500 osteoarthritis patients.
Since 2013, more than 1500 patients have been treated for OA using Magellan-manufactured autologous and allogeneic stem cell lines. Magellan, with its research collaboration partner Melbourne Stem Cell Centre Research, has published in several prestigious international medical journals. Overviews and links to the research are available on the Research page.
Magellan research is ongoing. Following the results of its Phase I/II allogeneic OA trial, these results were published in Osteoarthritis and Cartilage Open. Magellan is planning further research, including a Phase III allogeneic OA trial.
Since 2013, more than 1500 patients have been treated for OA using Magellan-manufactured autologous mesenchymal stem cell (MSC) lines. Magellan’s optimum MSC cell lines have been selected based on superior patient clinical outcomes and detailed genetic and DNA analysis of the characteristics of these optimum cell lines.
According to the National Health Survey 2017-2018, more than 2.2 million Australians suffered with OA in 2016.
One in five Australians aged over 45 have OA.
Although OA affects people of all ages, the prevalence increases sharply from 45 years, with up to 80 per cent of people over the age of 65 having radiological (scans) evidence of OA.
More than 500 million people across the world suffer from OA, which is recognised as a global unmet clinical need.
Magellan does not treat patients directly, but does supply the MSC’s to be used for OA patient treatments. For those seeking more information on OA treatment, please contact our collaborative clinical research partner, Melbourne Stem Cell Centre Research.
Magellan’s successful Phase I/II allogeneic OA trial results (2020) showed:
- long-term pain reduction;
- significant functional activity improvement; and
- evidence of osteoarthritis disease modification, cartilage regrowth, delay in OA progression with the potential to defer joint replacement surgery.
The results of the Phase I/II trial were published in Osteoarthritis and Cartilage Open.
Since 2013, Magellan – in partnership with Melbourne Stem Cell Centre Research – has undertaken a lengthy research and development pathway including clinical trial programs and, critically, real-world treatment case studies/series on the use of MSC’s for OA.
This work was based on Magellan’s preclinical research evidence in 2010 with veterinary osteoarthritis treatments. Magellan is well advanced in its medical and scientific product development strategy. This is supported by strong financial and corporate governance programs in order to commence its pivotal Phase III allogeneic OA trial in 2025.
Magellan’s plan is to continue to develop the company from a focus on R&D to a full scale diverse product commercialisation company across global markets.
Based on a successful Phase III allogeneic OA trial outcome, Magellan plans to make an application to the TGA for product approval and registration in Australia in 2028.
Magellan has partnered/ conducted extensive preclinical research. Its research partners have included: Australian Veterinary Stem Cell P/L; Swinburne University of Technology: Hudson Institute; and Monash Vet Clinic. A summary of the preclinical research is published in Stem Cells International.
To be confirmed
Magellan is a private Australian company. It first raised Capital in 2012 to commence the supply of autologous MSC’s to clinicians in Australia and New Zealand.
Magellan then raised further share capital in 2022 and 2023 to help fund its program of Phase I/II allogeneic OA trials, construct, expand and equip its GMP lab capabilities, commence overseas regulatory processes and develop its in-house management team.
Further completed Capital raise programs in 2024 and 2025 will enable Magellan to progress to the commencement of its pivotal Phase III allogeneic OA trial in 2025 and further develop its GMP lab and in-house management team resources.